if you added thymol blue to a solution of ph 5 what color would you observe
Reagents
- Sodium carbonate
- Sodium hydrogen sulfate
- Thymol blue
Safety
- Put on protective gloves and eyewear.
- Behave the experiment on the plastic tray.
General rubber rules
- Do not let chemicals to come into contact with the optics or mouth.
- Proceed immature children, animals and those not wearing heart protection away from the experimental area.
- Store this experimental set up out of attain of children nether 12 years of age.
- Clean all equipment afterward apply.
- Brand sure that all containers are fully airtight and properly stored after apply.
- Ensure that all empty containers are disposed of properly.
- Do non use whatsoever equipment which has not been supplied with the set or recommended in the instructions for use.
- Exercise not replace foodstuffs in original container. Dispose of immediately.
General commencement assist information
- In instance of eye contact: Wash out eye with plenty of h2o, property center open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Practise not induce vomiting. Seek firsthand medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash afflicted area with plenty of h2o for at to the lowest degree 10 minutes.
- In example of doubt, seek medical advice without filibuster. Take the chemical and its container with you.
- In case of injury always seek medical advice.
Advice for supervising adults
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for employ only by children over 12 years.
- Considering children's abilities vary so much, fifty-fifty within age groups, supervising adults should exercise discretion every bit to which experiments are suitable and condom for them. The instructions should enable supervisors to appraise any experiment to plant its suitability for a particular kid.
- The supervising developed should discuss the warnings and prophylactic information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a h2o supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the form of ane experiment, i.eastward. subsequently opening the bundle.
FAQ and troubleshooting
How much water should I cascade into the Petri dish?
Add together only every bit much water as needed to embrace the bottom of the Petri dish. Be careful non to add too much – you'll mix the contents of the Petri dish as part of the next step.
The solution didn't turn blue. What should I practise?
Add a few more drops of Na2CO3 solution and stir.
I poured too much thymol blue into the Petri dish. What should I do?
Don't worry, this isn't critical. Simply continue the experiment.
I yet have leftover thymol blue, merely I've run out of the other ingredients. What can I use to substitute for them?
Y'all can use various water-soluble substances of varying acidity to create patterns just like in our experiment. For instance, a solution of regular baking soda has an element of group i pH (i.e. the solution is bones). When making this solution, utilise hot h2o to assistance the baking soda dissolve more easily.
You can besides employ smelling salts which, when dissolved in water, form an ammonia solution, which is also a basic medium. However, this solution exudes an aggressive odor, so it'southward best to work with information technology in a well-ventilated area or fifty-fifty outside.
Meanwhile, you tin use solutions of citric or acetic acid or add some lemon juice to serve as acidic media.
Step-by-step instructions
Prepare a diluted sodium carbonate solution. This is an alkaline metal solution—its pH is higher than neutral.
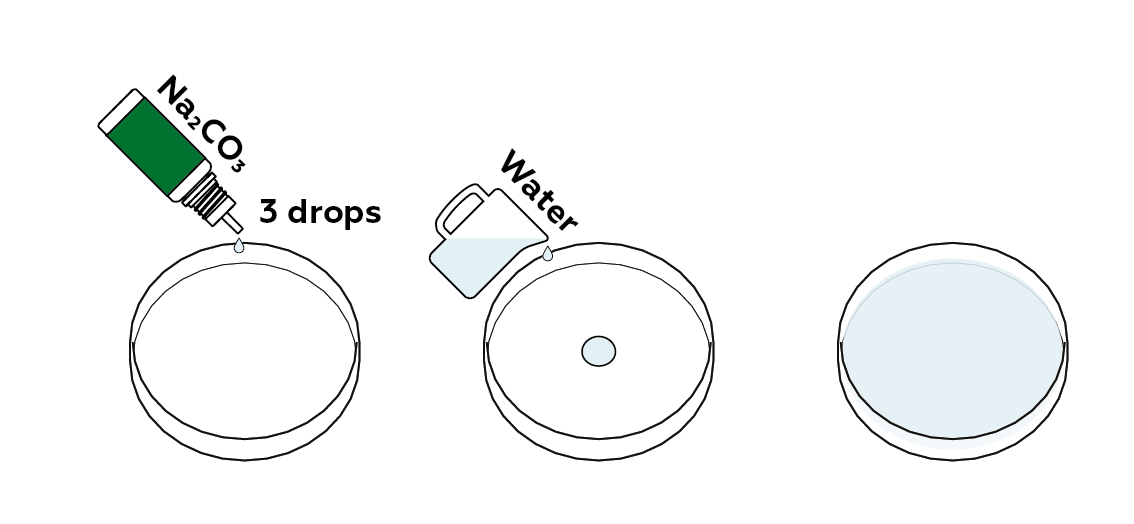
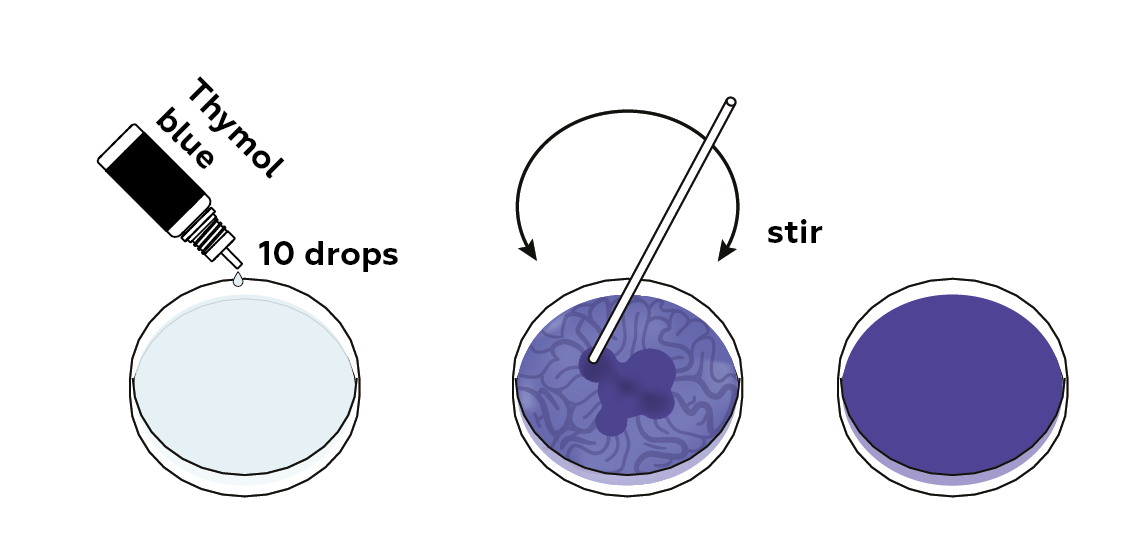
Add together the pH indicator, thymol blueish. It will turn blue in an alkaline metal medium.
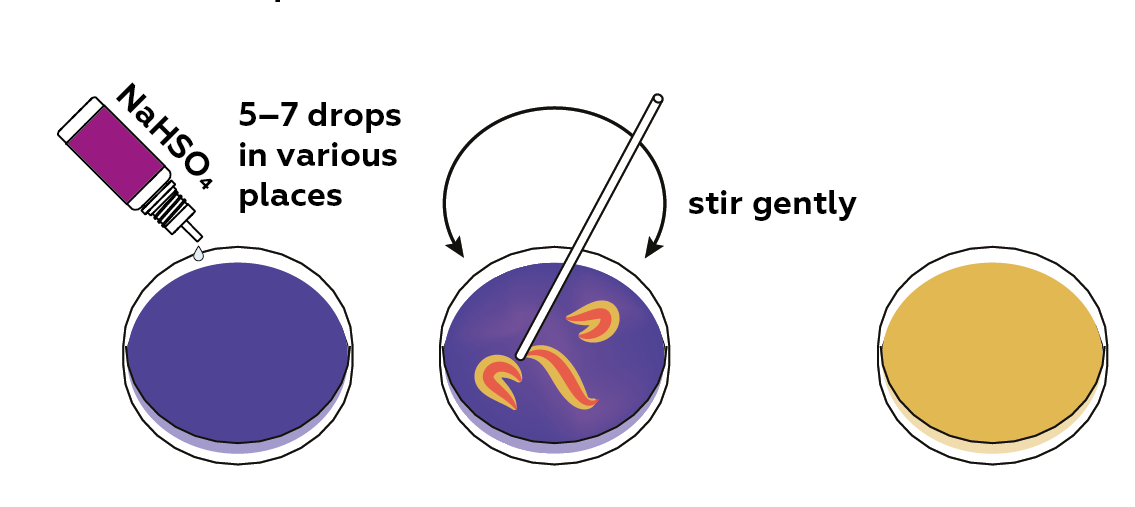
Now, add together sodium hydrogen sulfate NaHSO4 (an acrid salt) to lower the pH of the solution.
At present, add sodium carbonate Na2COthree (an alkaline solution) to raise the pH of the solution.
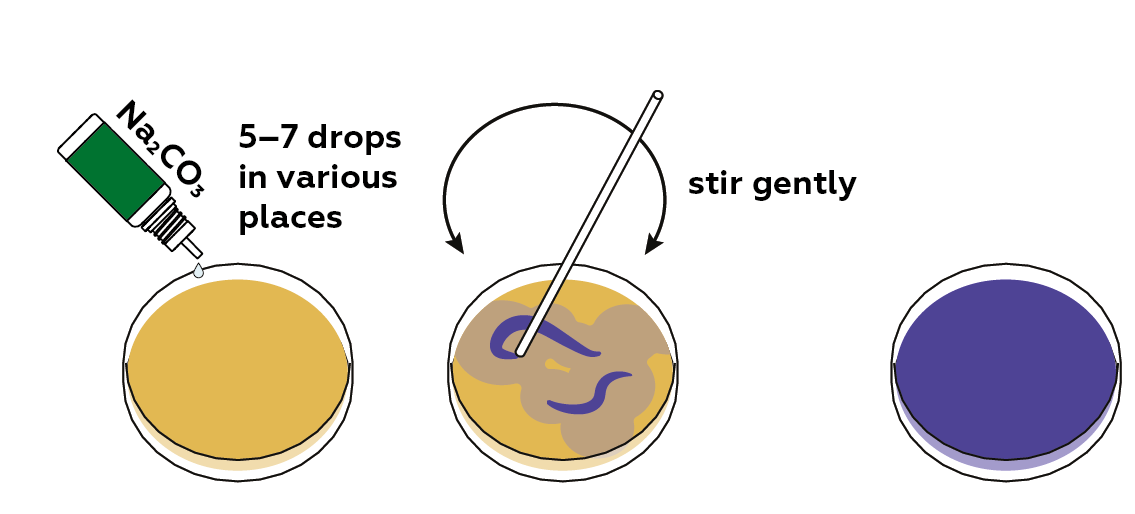
You tin can repeat steps three and four several times. Try calculation varying volumes of NaHSO4 and NaiiCO3.
Expected result
Thymol blue turns bluish when added to a bones medium. If an acidic solution of sodium hydrogen sulfate is added, and the mixture is stirred gently, orangish patterns will appear in the Petri dish.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste material with household garbage. Cascade leftover solutions downward the sink. Launder with an backlog of water.
Scientific description
Why does thymol blue change color?
Thymol blue changes color depending on the medium it is in. In other words, it is a pH indicator. In a basic medium, it turns blue; in an acidic medium, it turns red; in an about neutral medium, it turns yellow.
Blue, red, and yellowish are considered 'primary' colors because combining them can yield many additional variations: blue + yellow = green, yellow + ruddy = orange, red + blue = violet. You can create these combinations in your Petri dish too! Only stir the contents gently with a wooden stick.
How does the acerbity of the solution in the Petri dish change?
The acerbity of a solution depends on its ratio of positively-charged hydrogen ions H+ (protons) to negatively-charged hydroxide ions OH-. A solution is acidic if it contains more H+ ions than OH- ions; conversely, it is basic if it contains more OH- than H+.
Ordinary water is practically neutral: the numbers of H+ and OH- in it are well-nigh equal. However, some substances can increase the concentration of H+ or OH- ions, thus increasing or decreasing the pH respectively.
For instance, sodium carbonate, which we added to the Petri dish commencement, creates a basic medium. Its interaction with water produces a large amount of hydroxide ions OH-:
Na2CO3 + HtwoO → 2Na+ + HCOthree - + OH-
This abundance of hydroxide ions makes the solution in the Petri dish basic. Thymol blue can be used to confirm this – information technology turns blue when added to the solution.
Sodium hydrogen sulfate, in contrast to sodium carbonate, dissolves in h2o and releases protons H+:
NaHSOfour → Na+ + H+ + SO4 2-
The solution becomes acidic as soon as sodium hydrogen sulfate is added to the Petri dish.
Every bit we add reagents to unlike places in the Petri dish, the solution's acerbity changes unevenly. In some places, H+ ions dominate, while OH- reign in others. As a outcome, the thymol blue peppers the solution with a variety of colors. When mixed, the acidity of the medium becomes uniform all over the solution, so the thymol bluish'south color becomes uniform as well.
That's interesting!
How do pH levels bear on living organisms?
Changes in the pH of various solutions effectually or even within living organisms can strongly affect their quality of life. For instance, a pH below 6 is usually too acidic for fish. Even if it won't kill fish and other aquatic species outright, it tin interfere with their growth and reproduction. Fish eggs will not hatch if the pH of the water is beneath five. On the other hand, if the pH rises in a higher place nine, fishes' ability to absorb oxygen worsens, and they can even suffocate.

It is also important to monitor the pH of rainwater, which can be quite acidic due to the presence of sulfuric acid H2SO4 and nitric acid HNO3. These acids class in the atmosphere from nitrogen and sulfur oxides, which are emitted as waste past numerous industries, transport systems, boilers, and thermal power plants. Acrid rain with a low pH (less than 5.half dozen) destroys flora and sea life. Thereafter, in acidic soil, trees and plants are less able to absorb the groundwater they demand to abound.
pH is as well of import for human beings, as the body maintains a certain acid-base of operations balance. Disturbances in this balance can lead to many serious ailments. Each individual organ or bodily fluid (such as blood or saliva) maintains a certain pH. The stomach, for case, houses an extremely acidic medium (pH 1.8 – iii.0). This medium is maintained by hydrochloric acid HCl, which promotes the breakdown of food in the breadbasket and acts as an antibacterial agent. The digestive products then enter the intestine, where the pH is alkaline (pH 7 – eight). Thus, acid is formed in one area of the stomach and neutralized in the other (at the bottom of the tum):
HCl + NaHCOiii → NaCl + CO2 + H2O
Stomach acid is mainly neutralized by sodium bicarbonate NaHCO3.
Source: https://melscience.com/US-en/chemistry/experiments/thymol-blue-v2_petri-dish/
0 Response to "if you added thymol blue to a solution of ph 5 what color would you observe"
Postar um comentário